Lessons in Antisense
Lesson 16 – PS ASO-induced PS ASO-RNP Aggregates
August 4, 2025 by Dr. Stan Crooke

Introduction
Though some intracellular organelles are enclosed in membranes, many are not, including nucleoli, paraspeckles, processing bodies (P-bodies), Cajal bodies (CBs), stress granules and others. In simple terms, these structures are all aggregates of RNAs and proteins. RNAs appear to serve as scaffolds and, for each natural aggregate, there are core proteins that must be incorporated into the aggregate as well as many ancillary proteins that may associate transiently or under some circumstances. Each of the naturally occurring aggregates serves important functions in the cell and can undergo phase transitions. Simply put, natural aggregates can exist as “liquids” or semi-liquids or “solids”. In the “liquid” state, proteins and RNAs can exchange freely, resulting in the dynamic interplay between the functional aggregates and the cellular environment necessary to respond to changes in the environment and maintain homeostasis. In the “solid” state, exchanges with the cellular environment are significantly more limited and may limit or disrupt the function of these aggregates.
My lab has demonstrated that PS ASOs can also serve as scaffolds and form a remarkable number of aggregates in both the nucleus and the cytoplasm. Surprisingly to me, we have shown that a 20-mer PS ASO can displace NEAT1, a several thousand nucleotide long noncoding RNA, from paraspeckles and aggregates that resemble core paraspeckles and that can participate in RNA splicing, which is the main function of paraspeckles. PS ASOs can also form functional P-bodies and stress granules in the cytoplasm.
The formation of each type of aggregate by PS ASOs is ASO concentration and time dependent. Preliminary data suggest that aggregate formation may be affected by the hydrophobicity of the 2′-modification in the PS ASO and sequence, but the structure–activity relationships are poorly understood. Some of these aggregates form at therapeutic concentrations while others form at cytotoxic concentrations. In the table below, I summarize the aggregates we have identified, the concentrations, and times after introduction of PS ASOs at which they form, and what we know about the compositions of the aggregates.
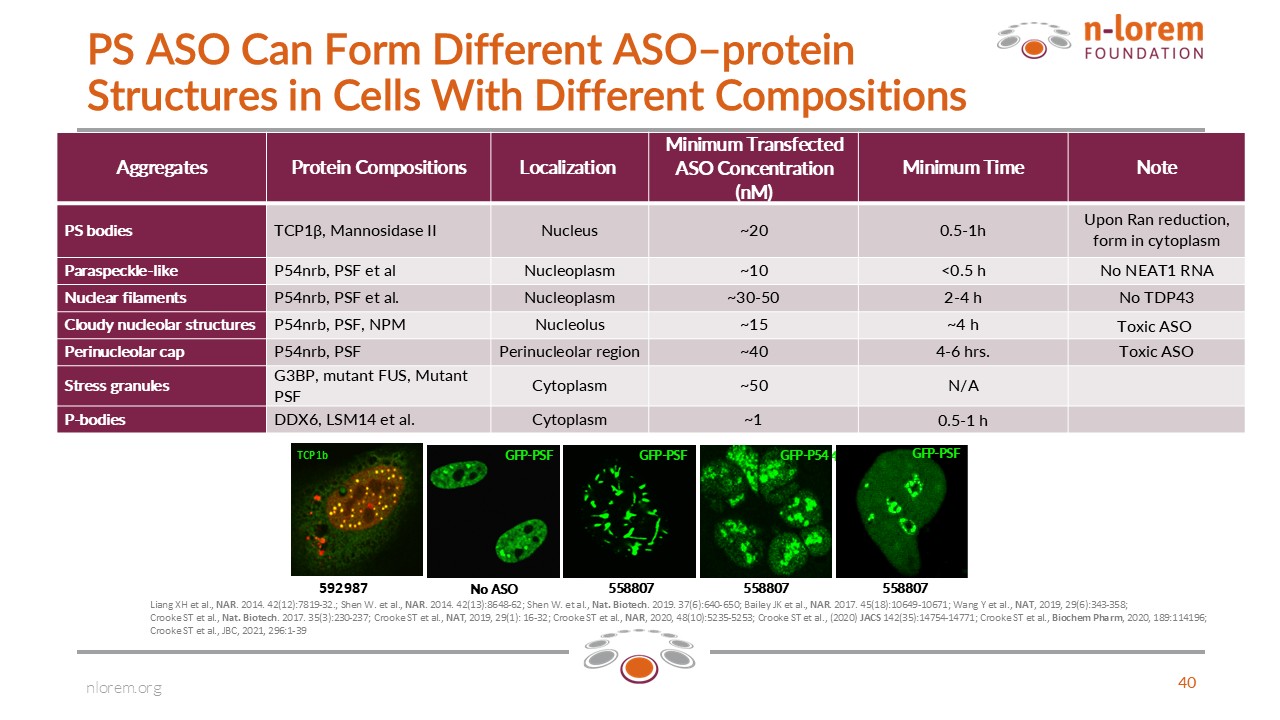
The simplest of the PS ASO-induced aggregate is nuclear PS bodies. We have identified only two proteins in PS bodies, TCP1 beta and mannosidase 2. Under some cytotoxic conditions, PS body-like structures can form in the cytoplasm, and these are more complex and comprised of different proteins. We have used fluorescence quenching methods to demonstrate that PS ASO-induced aggregates interact dynamically with the cellular environment, exchanging both some proteins and RNAs. We have also shown that PS ASO-induced aggregates (or biomolecular condensates as naturally occurring aggregates are called) can undergo phase separation, and when cytotoxic nucleolar mis-localized paraspeckle-like aggregates undergo solid phase separation, they no longer exchange multiple proteins with the nucleoplasm, nor are they cytotoxic. Remarkably, when we established toxic PS ASO-resistant cells, we observed that PS ASO-induced aggregates underwent solid phase separation and formed function micro-nucleolar like structures that transcribed and processed tiny amounts of pre-rRNA.
Though most PS ASO-induced aggregates are benign, toxic PS ASOs of all 2′ chemical classes studied bind more tightly to RNase H1 and disrupt the structure of RNase H1 more significantly than non-cytotoxic PS ASOs. The PS ASO bound RNase H1 then binds paraspeckle proteins and a variety of RNAs, mis-localizes to nucleoli, and disrupts nucleolar function causing apoptosis. We believe this mechanism accounts for the cytotoxicity of toxic PS ASOs of all chemical classes.
Conclusions
In conclusion, it is clear that PS ASOs can cause the formation of multiple cellular aggregates at therapeutic and cytotoxic concentrations. Most of the aggregates appear to be benign, but some certainly are cytotoxic. PS ASO-induced aggregates interact dynamically with the cellular environment and undergo phase separation and, at least in the case of cytotoxic nucleolar aggregates, phase separation is cytoprotective. As exciting as what we have learned is, clearly, we have vastly more questions than answers. What are precise compositions of each aggregate, how do compositions vary over time or as a function of cell type, what is the precise order of addition of core components of each aggregate, what are the structures of the aggregates, and what are the structure-activity relationships of PS ASO aggregate formation? These are a few of the questions that need to be addressed. Though I cannot speak definitively, I believe that this is a vital area of research that could yield major advances in the technology.

We cannot do
this alone
Together we are changing the world—
one patient at a time
We hope that you join us on this journey to discover, develop and provide individualized antisense medicines for free for life for nano-rare patients. The ultimate personalized medicine approach – for free, for life.
Follow us on social for updates on our latest efforts


