Lessons in Antisense
Lesson 4 – The Magic of Phosphorothioates (PS)
May 4, 2025 by Dr. Stan Crooke

Introduction
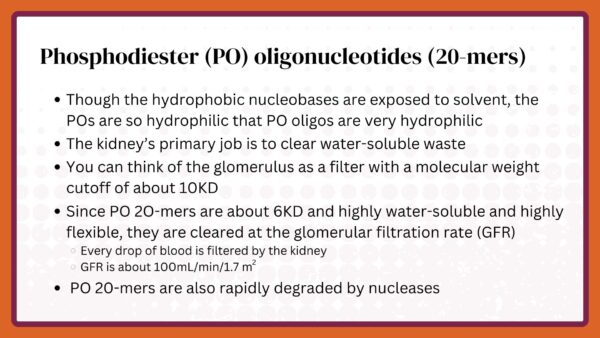
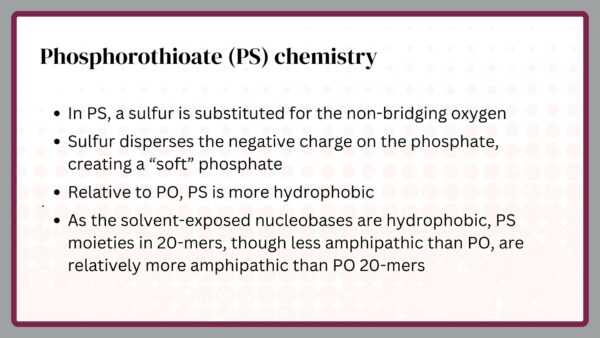
Arguably, the most important chemical modification of ASOs was created by Fritz Eckstein some years before Ionis and other antisense companies were formed. He substituted a sulfur for the non-bridging oxygen in the phosphate moiety. The sulfur dispersed the negative charge on the phosphate making it more lipophilic (fat liking). The modification was called phosphorothioate (PS). Since the phosphate links each nucleotide in natural nucleotides, it was obvious that PS might be used to link nucleotides in an ASO.
When Ionis was formed, there were two neutral modifications, methylphosphonate and morpholino, and one charged, phosphorothioate. I selected phosphorothioate because I believed that ASOs would need to be amphipathic, i.e. display a charge distribution that would allow them to be soluble both in water and lipids. I worried that lipophilic ASOs, such as methyphosphonate and morpholino, would become trapped in membranes of cells and various fat depots in the body.
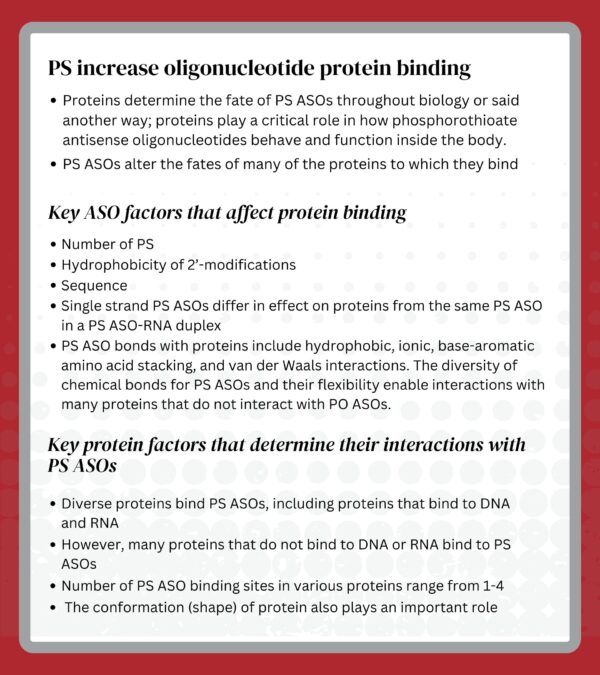
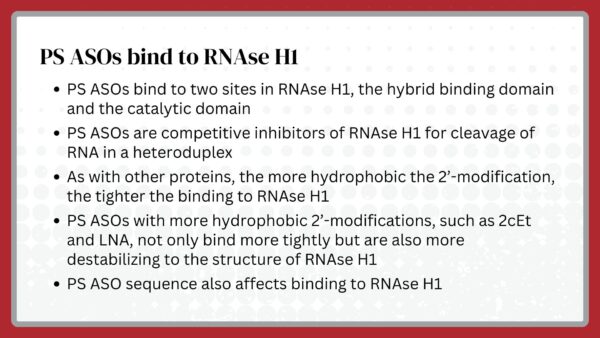
That turned out to be an important decision, but only partly because of the amphipathicity of PS. In fact, as we have learned more about the behavior of PS ASOs, we discovered that, in addition to providing some enhanced stability, PS dramatically changed protein binding compared to phosphodiester (PO). Though we have invested many years in trying to find a substitute for PS, to date, no modification has yielded the behavior of PS ASOs.

Conclusions
Though the choice to pursue PS as the backbone was not obvious, that choice has proven to be critical, but not entirely for the reasons PS was originally selected. Certainly, the amphipathicity afforded by PS modifications and the ease of synthesis proved to be important, but the single most important feature of a PS backbone, protein binding, was a surprise. Not only was the extent of protein binding a surprise, but also was the diversity of proteins with which PS ASOs interact. The importance of those interactions and the sensitivity of protein binding to sequence, the hydrophobicity of 2’-modifications, and the number and placement of PS moieties proved to be critical.
Longer term, learning that protein interactions determine the fates of PS ASOs throughout biology was an exciting event. It suggested that learning to manipulate protein binding via modification of some sites in a PS ASO was feasible and important. As we learn more about how to take advantage of protein binding, we may look forward to true “designer PS ASOs”.

We cannot do
this alone
Together we are changing the world—
one patient at a time
We hope that you join us on this journey to discover, develop and provide individualized antisense medicines for free for life for nano-rare patients. The ultimate personalized medicine approach – for free, for life.
Follow us on social for updates on our latest efforts


